BCM 441 LITERATURE SPOTLIGHT
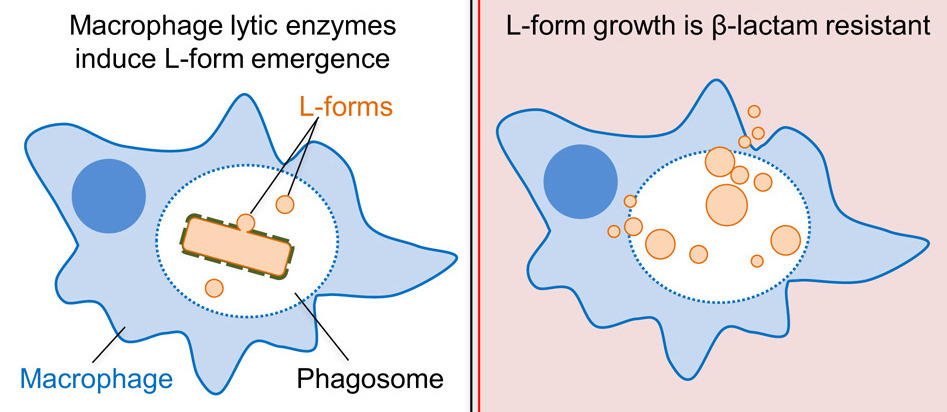
β-lactams are a prominent class of antibiotics including Penicillin which inhibit peptidoglycan cell wall synthesis in bacteria, leading to cell death. Paradoxically, macrophage lysozyme supports emergence of a β-lactam resistant L-form morphology which can survive and proliferate without a cell wall.
Antibiotics have undoubtedly altered the course of modern medicine, but the powerful compounds often praised as miraculous are not as infallible as some like to believe.2 The recent prominence of antibiotic resistance has led to a greater focus on the shortcomings and misuse of antibiotics.2 Understanding why and when antibiotic treatment will fail is of significant clinical importance, as antibiotics are still some of the most frequently prescribed and valuable medications today.
Bacterial cell walls contain the carbohydrate and amino acid polymer peptidoglycan (PG).1,3 Peptidoglycan synthesis is a multistep process occurring both inside and outside of the cell. As discussed in lecture, it begins with synthesis of a PG precursor, Lipid II, by Mur A, B, C, D, E, and F enzymes in the cytoplasm to generate a peptide-conjugated UDP-MurNAc molecule, which is then conjugated to an undecaprenyl unit in the membrane by MraY. MurG then attaches a GlcNAc, forming Lipid II. Lipid II is then flipped to reside on the surface of the cell, where transpeptidases build the peptidoglycan network through a series of nucleophilic acyl substitutions, forming long carbohydrate polymers linked by short peptides.1,3
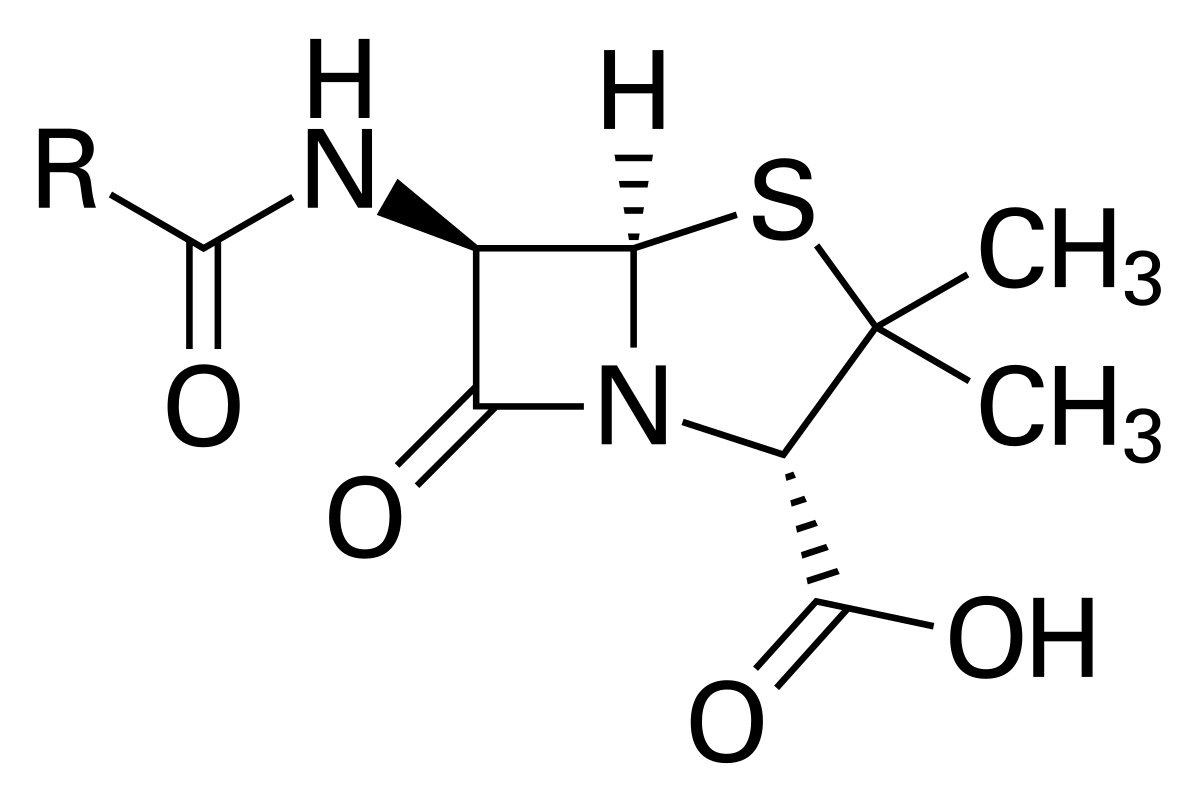
Many antibiotics inhibit enzymatic steps involved in Lipid II synthesis, ultimately interfering with the cell wall and resulting in cell death.1,3 Perhaps even more common, though, are antibiotics which interfere with the processes occurring on the outside of the cell, including β-lactams.1 β-lactams, including Penicillin (Fig. 1), interfere with peptidoglycan synthesis by covalently modifying the pencillin binding protein transpeptidases (PBPs) essential for polymer formation, typically resulting cell death.
Kawai et al.1 identified an interesting connection between such antibiotics and a peculiar bacterial morphology known as the L-form. L-form bacteria lack the typically essential and characteristic cell wall, and undergo an odd heterogenous proliferation driven by changes in membrane growth.6 More specifically, L-form progeny “bleb” off from the rod-structured (walled) parent cell (Fig. 2) when membrane growth excessively surpasses that of the cytoplasm (the L-form is associated with increased fatty acid synthesis),6 but certain conditions are required for these cells to escape their cell walls in the first place – often referred to as the L-form switch.
These authors previously demonstrated that upregulation of autolytic activity in the cell wall (via WalR) promotes this L-form escape from the walled morphology.5 L-form bacteria can continue to proliferate without a cell wall.6 Thus, they confer resistance to many common antibiotics because they do not require peptidoglycan synthesis.1
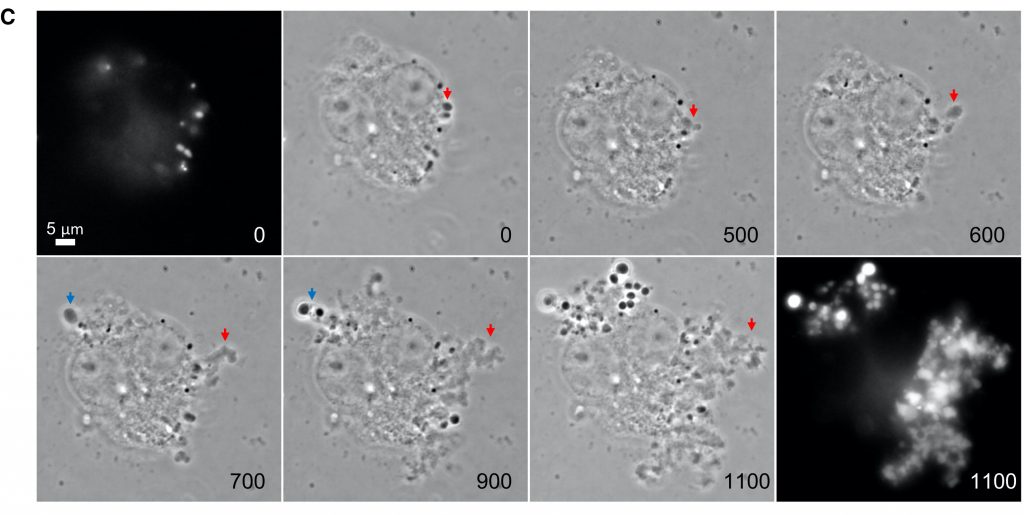
Antibiotics which inhibit Lipid II synthesis are known to induce L-form switch in many bacteria. β-lactam treatment had the opposite effect, inhibiting transition to the L-form state.1 The authors investigate the apparent difference in efficacy of Lipid II synthesis inhibitors versus PG assembly inhibitors in conferring the L-form switch, and find that antibiotics which interfere with class A PBPs appear to additionally block autolytic enzyme activity necessary L-form release. Experiments using time-lapse microscopy allow the authors to visualize the L-form switch under a variety of experimental conditions (i.e. Fig. 2), and they demonstrate that while L-form growth is typically prevented in the presence of PenG (a penicillin/β-lactam), it can be recovered with the addition of exogenous lytic enzyme.
The authors then put these initial findings in conversation with physiological systems by showing that host lytic enzymes can promote the L-form switch. Host macrophages were found to contain lysozyme, an antimicrobial immune effector which catalyzes the breakdown of PG as part of the innate immune response.8 The L-form switch can be observed in the macrophage-engulfed cells by microscopy, and strikingly, the L-forms can escape the macrophage and continue to proliferate even once the macrophage has died (Fig. 3). Further, the L-form morphology confers complete resistance to β-lactam antibiotics. This challenges the widely held assumption that the majority of macrophage-pathogen interactions necessarily end in pathogen destruction. Kawai and colleagues suggest that this likely accounts for the majority of lytic activity necessary for L-form switch in physiological systems, though some L-form activity is still observed in the presence of lysozyme inhibition, likely due to other, unidentified immune effectors with lytic function.
They take this further to see if host macrophages can actually provide a protective environment to gram-positive bacteria in the presence of β-lactam antibiotics. Remarkably, the investigators demonstrate that not only can these cells survive and continue to proliferate, but they can return to their walled morphology once the antibiotic is removed, as evidenced by cell growth on hypotonic media, where L-form cannot survive. Thus, this antimicrobial immune cell provides paradoxical shelter from antibiotic action, as the cell-wall deficient morphology can retain viability inside the macrophage.

The authors highlight the possible implications of these findings in understanding recurrent infections, providing translational potential and speaking in part to the impact of the work. They suggest that typical physiological conditions would be sufficient to support L-form growth during antibiotic treatment, and that the walled morphology could subsequently reemerge. Unlike other cell states implicated in recurrent infection,1 L-forms can not only survive in the presence of antibiotics, but can proliferate as well.
Kawai and colleagues work raises a number of questions, many of which they address in their discussion. Most notably, the question of what characteristics allow long term persistence of the L-form morphology and precisely how long this can occur in in vivo niches (such as macrophages) certainly warrants further study. This will be important to truly understanding the physiological and clinical applications of this work. Further clinical study will also be necessary to understand why and when L-forms may emerge, as β-lactams are widely used and typically effective. The authors suggest this may be more common in those with weakened immune systems, but this explanation seems insufficient given the critical role of the immune system in conferring the L-form morphology elucidated in this paper. Since the publication of this article, this group published a more clinically driven analysis of the role of L-forms in recurrent urinary tract infections in Nature Communications which has started to address some of these questions.8
Despite the questions that remain, Kawai et al. provide fascinating insight to the paradoxical role of the innate immune system in β-lactam antibiotic efficacy. Understanding the complex mechanisms impacting antibiotic evasion is essential for safe use and systemic design of antibiotics.
Works Cited
- [Original Paper] Kawai, Y.; Mickiewicz, K.; and Errington, J. Lysozyme Counteracts β-Lactam Antibiotics by Promoting the Emergence of L-Form Bacteria. Cell. 2018, 172(5): 1038-1049. https://doi.org/10.1016/j.cell.2018.01.021
- Aminov, R.I. A Brief History of the Antibiotic Era: Lessons Learned and Challenges for the Future. Front Microbiol. 2010, 1:134. https://dx.doi.org/10.3389%2Ffmicb.2010.00134
- Kouidmi, I.; Levesque, R.C.; Paradis-Bleau, C. The biology of Mur ligases as an antibacterial target. Molecular Microbiology. 2014, 94(2): 242-253. https://doi-org.muhlenberg.idm.oclc.org/10.1111/mmi.12758
- Wikipedia. Penicillin (Image). https://en.wikipedia.org/wiki/Penicillin (Accessed Apr 19, 2020).
- Domínguez-Cuevas, P.; Mercier, R.; Leaver, M.; Kawai, Y.; and Errington, J. The rod to L-form transition of Bacillus subtilis is limited by requirement for the protoplast to escape from the cell wall sacculus. Molecular Microbiology. 2012, 83(1): 52-66. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2958.2011.07920.x
- Mercier, R.; Kawai, Y.; and Errington, J. Excess Membrane Synthesis Drives a Primitive Mode of Cell Proliferation. Cell. 2013, 152(5): 997-1007. https://doi-org.muhlenberg.idm.oclc.org/10.1016/j.cell.2013.01.043
- KEGG. Enzyme 3.2.1.17 (Lysozyme). https://www.kegg.jp/dbget-bin/www_bget?ec:3.2.1.17 (Accessed Apr 19, 2020).
- Mickiewicz, K.M.; Kawai, Y.; Drage, L.; Gomes, M.C.; Davidson, F.; Pickard, R.; Hall, J.; Mostowy, S.; Aldridge, P.D.; and Errington, J. Possible role of L-form switching in recurrent urinary tract infection. Nature Communications. 2019, 10, 4379. https://doi-org.muhlenberg.idm.oclc.org/10.1038/s41467-019-12359-3